All medications cause side effects, but thiazide diuretics, of which hydrochlorothiazide is one, are among the better tolerated of the antihypertensive agents.1-3 In studies, people are less likely to withdraw due to adverse effects of HCTZ than from placebo.2,3
A 2014 Cochrane Review meta-analysis found 60 randomized controlled trials that studied the dose-related trough blood pressure-lowering efficacy of 6 different thiazide diuretics in 11 262 participants.3 Compared with placebo, HCTZ had significantly lower withdrawals due to adverse effects (relative risk [RR] 0.64, 95% confidence interval [CI] [0.43 – 0.93]) based on 20 trials (n=3698).
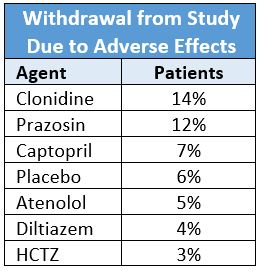
A randomized, double-blind study of 1292 hypertensive men (mean age 59±10 years; 48% black) compared six antihypertensive drugs and placebo for efficacy and tolerability.2 It found the withdrawal rate due to subjective side effects for patients on HCTZ was less than all other drugs. No side effects occurred more frequently in patients taking HCTZ than placebo.
Side Effect Symptoms
According to the FDA-approved prescribing information for HCTZ, the most common side effects reported are listed by severity, but not by frequency.4 Therefore, this information is not particularly useful for discussing common symptoms. There are many studies with varying incidence of side effects, but few that compare HCTZ monotherapy to placebo.
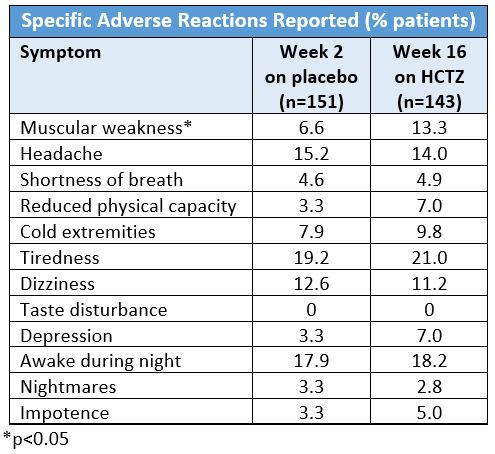
Helgeland et al. performed a double-blind randomized trial of 436 hypertensive patients (age 20 – 75 years) comparing the efficacy and tolerability of HCTZ (25 mg/day; n=151), enalapril (20 mg/day; n=140), and atenolol (50 mg/day; n=145).5 The 16-week study monotherapy treatment period was preceded by a two-week placebo run-in period. The patients were also surveyed with a form to report complaints and questioned about specific symptoms of discomfort at the end of the two-week placebo period and at the end of the treatment period. None of the symptoms in people taking HCTZ at week 16 reached statistical significance except muscle weakness. The results for HCTZ are as follows:
A randomized, double-blind 48-week trial which compared the efficacy and tolerability of HCTZ (12.5 – 25 mg/day; n=215), atenolol (25 – 50 mg/day; n=215), nitrendipine (10 – 20 mg/day; n=218), and enalapril (5 – 10 mg/day; n=220) in hypertensive patients found that in the 48 weeks four patients (1.8%) withdrew from the study due to gastrointestinal complaints and two (0.9%) for muscle cramps.6
A 1990 study comparing the efficacy of low-dose (25 – 50 mg) and high-dose (50 – 100 mg) HCTZ daily in 690 older hypertensive men (>60 years) found no difference in the incidence of side effects between doses.7 Side effects were “uncommon”. Though the authors did not give specific data, they state that the prevalence of side effects in order of increasing prevalence were weakness, muscle cramps, abdominal pain, constipation, and nocturia. The men did not experience a change in sexual function.
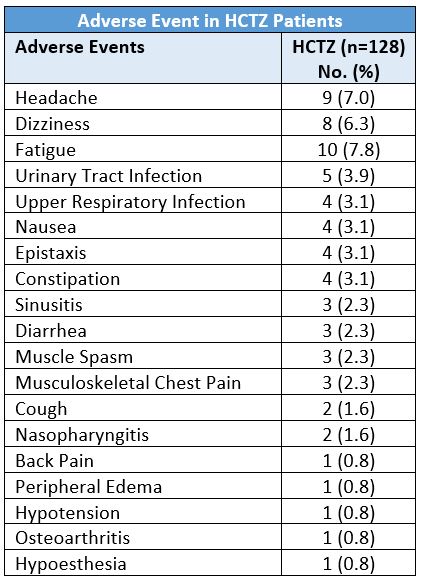
A 2011 16-week trial evaluated the safety and efficacy of HCTZ (12.5 mg/day; n=128) and valsartan monotherapies versus single-pill valsartan/HCTZ in 384 hypertensive, very elderly (≥70 years) patients.9 At week 16 the adverse events which occurred in more than two percent of the patients in the HCTZ monotherapy group were:
References
- Ramsay LE. Thiazide diuretics in hypertension. Clin Exp Hypertens 1999; 21 (5-6): 805-814.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328 (13): 914-921.
- Musini VM, Nazer M, Bassett K, et al. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev 2014; (5): Cd003824.
- Hydrochlorothiazide [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc.; 2011.
- Helgeland A, Strommen R, Hagelund CH, et al. Enalapril, atenolol, and hydrochlorothiazide in mild to moderate hypertension. A comparative multicentre study in general practice in Norway. Lancet 1986; 1 (8486): 872-875.
- Philipp T, Anlauf M, Distler A, et al. Randomised, double blind, multicentre comparison of hydrochlorothiazide, atenolol, nitrendipine, and enalapril in antihypertensive treatment: results of the HANE study. HANE Trial Research Group. BMJ 1997; 315 (7101): 154-159.
- Materson BJ, Cushman WC, Goldstein G, et al. Treatment of hypertension in the elderly: I. Blood pressure and clinical changes. Results of a Department of Veterans Affairs Cooperative Study. Hypertension 1990; 15 (4): 348-360.
- Pareek AK, Messerli FH, Chandurkar NB, et al. Efficacy of low-dose chlorthalidone and hydrochlorothiazide as assessed by 24-h ambulatory blood pressure monitoring. J Am Coll Cardiol 2016; 67 (4): 379-389.
- Izzo JL, Jr., Weintraub HS, Duprez DA, et al. Treating systolic hypertension in the very elderly with valsartan-hydrochlorothiazide vs. either monotherapy: ValVET primary results. J Clin Hypertens (Greenwich) 2011; 13 (10): 722-730.
.png)




